J&J Coronavirus Shot No Longer Available in the U.S.
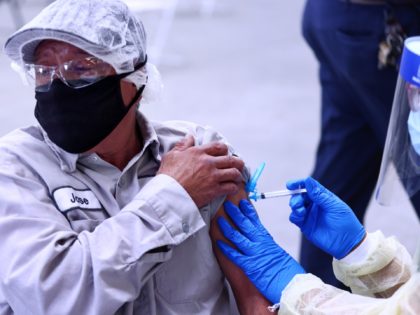
The non-mRNA coronavirus Johnson & Johnson shot is no longer available in the U.S., according to the U.S. Centers for Disease Control and Prevention (CDC).

The non-mRNA coronavirus Johnson & Johnson shot is no longer available in the U.S., according to the U.S. Centers for Disease Control and Prevention (CDC).

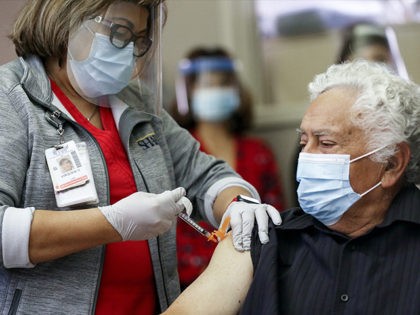
Texas Attorney General Ken Paxton on Monday launched an investigation into whether or not Pfizer, Moderna, and Johnson & Johnson misrepresented the efficacy and safety of their coronavirus vaccines to the public.

The Food and Drug Administration (FDA) has now approved the Moderna and Johnson & Johnson booster shots for the vulnerable.
Johnson & Johnson is seeking to use bankruptcy as a means to temporarily halt all lawsuits that allege plaintiffs developed cancer as a result of using the company’s baby powder products.

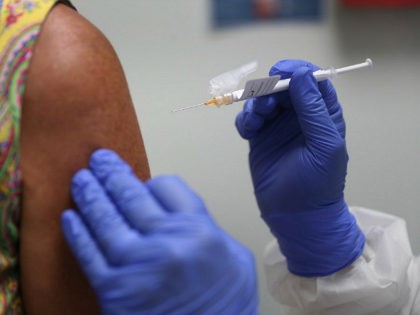
The Food and Drug Administration (FDA) is questioning the evidence provided by Johnson & Johnson in its application for authorization of its coronavirus booster shot, according to a document released prior to the October 15 meeting with FDA vaccine advisers.

President Joe Biden’s federal agencies are dodging on whether or not Afghans arriving in the United States are mandated to receive one of the three available coronavirus vaccines that millions of Americans are now being required to take.
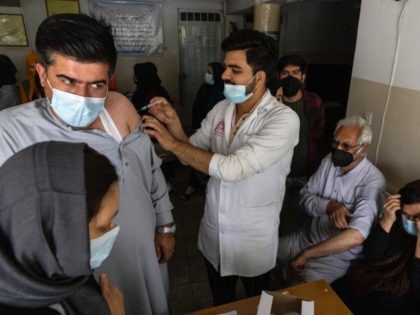
President Joe Biden’s administration is planning to give the Johnson & Johnson coronavirus vaccine to border crossers who arrive at the United States-Mexico border, sources say.

Pfizer representatives will meet with top U.S. health officials Monday to outline their plans to request federal authorization of a third dose of its coronavirus vaccine. The head-to-head comes as President Joe Biden’s chief medical adviser conceded “it is entirely conceivable, maybe likely” booster shots will be needed.

The Health Ministry of Brazil announced on Tuesday that it would suspend a contract with the Indian manufacturer Bharat Biotech to purchase 20 million doses of its coronavirus vaccine product after left-wing newspapers accused the government of conservative President Jair Bolsonaro of corruption.
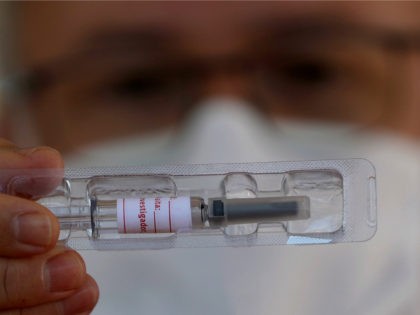
The United Kingdom’s medicines regulator has authorised the use of the Johnson & Johnson subsidiary Janssen’s single-shot vaccine, the fourth vaccine the nation has approved so far.
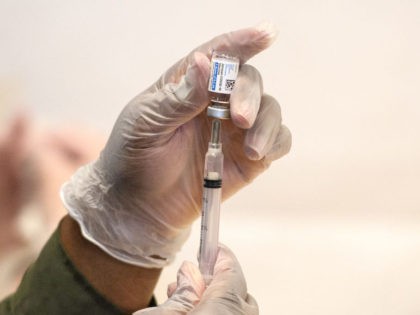
OTTAWA, Ontario (AP) – Plans to distribute the first 300,000 doses of the Johnson & Johnson COVID-19 vaccine in Canada next week are on hold after Health Canada learned part of them were manufactured at a Maryland facility that messed up the ingredients in 15 million doses bound for the U.S. market.
During a phone interview on Thursday with Fox Business Network’s “Mornings with Maria,” former President Donald Trump slammed the Food and Drug Administration’s (FDA) decision to temporarily pause the Johnson & Johnson COVID-19 vaccine due to concerns of blood clots.

Nearly three-quarters of unvaccinated Americans are unwilling to receive the Johnson & Johnson vaccine following U.S. health agencies calling for its pause last week over concerns of blood clots, a Washington Post-ABC News poll released Monday showed.
The Biden administration has ended the pause and will allow Johnson & Johnson to resume Chinese coronavirus vaccinations.
More than 170,000 people in the United States have died from the Chinese coronavirus during President Joe Biden’s first three months in office.

Many pro-life organizations have released information providing clarification about the use of abortion-derived fetal cell lines in the development, production, or testing of the different coronavirus vaccines.
Wednesday on MSNBC’s “Morning Joe,” White House chief medical adviser Anthony Fauci sounded off on the “immediate pause” in the rollout of Johnson & Johnson’s single-dose coronavirus vaccine. This comes as six Americans have reportedly developed blood clots after receiving the shot.

Tuesday on FNC’s “Tucker Carlson Tonight,” host Tucker Carlson opened his program by reacting to an announcement earlier in the day that health officials were recommending to halt the distribution of the Johnson & Johnson vaccine.

Florida Gov. Ron DeSantis (R) offered words of calm on Tuesday following U.S. federal health agencies calling for the “immediate pause” of administration of the Johnson & Johnson coronavirus vaccine, noting that no major issues with that particular vaccine have been reported in the Sunshine State.

U.S. health agencies on Tuesday will issue a joint call for Washington to begin an “immediate pause” in the rollout of Johnson & Johnson’s single-dose coronavirus vaccine.
“We remain on track to deliver a vaccine before the end of the year, and maybe even before November 1,” he said. “We think we can probably have it sometime during the month of October.”
A big surge in consumer spending on over-the-counter medicine and personal care products sent sales soaring.

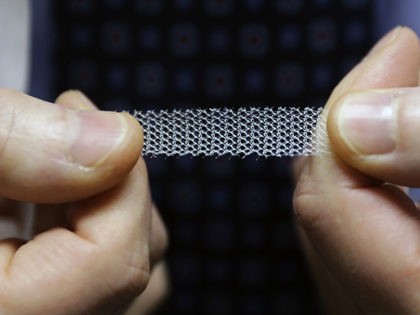
The state of Ohio announced Thursday that Johnson & Johnson has agreed to pay nearly $117 million to settle an investigation into their deceptive marketing of transvaginal mesh devices.
Johnson & Johnson was ordered to pay $8 billion to a man who grew breasts after taking the antipsychotic drug Risperdal, the company having failed to warn him about the side effects.

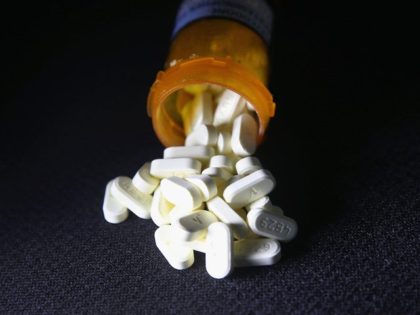
Cleveland County District Judge Thad Balkman ruled Monday that Johnson & Johnson must pay the state of Oklahoma $572,102,028 for its role in exacerbating the opioid crisis in the state.
The government of Uganda announced Monday it has begun trial use for an experimental Ebola vaccine by Johnson & Johnson amid the second-worst Ebola outbreak on record. Uganda accepted the vaccine after the Democratic Republic of Congo (DRC)’s former health minister resigned over alleged pressure to introduce it into that country’s population.

Democratic Republic of Congo (DRC) Health Minister Oly Ilunga resigned from his post Monday after being removed as the head of the nation’s Ebola outbreak response, a move that followed Ilunga rejecting pressure to introduce a second Ebola vaccine into the population.

Purdue has reportedly settled a lawsuit by the state of Oklahoma which accused them of fueling the opioid abuse epidemic.
MyPillow CEO Mike Lindell has taken to Twitter to assure customers that he will not be pulling his advertising from Laura Ingraham’s Fox News show despite the liberal outrage over her feud with anti-gun Parkland kid David Hogg.

Fox News has reiterated its support for conservative talk show host Laura Ingraham, as advertisers threaten to leave the show over criticisms she made of Parkland School shooting survivor David Hogg.
