The U.S. Centers for Disease Control and Prevention (CDC) said on Tuesday it is communicating with Chinese health officials about the explosion of pediatric respiratory diseases and pneumonia in northern China.
Senior Biden administration officials met with pharmaceutical companies on Monday to discuss manufacturing and distributing respiratory syncytial virus (RSV) immunizing products.
The White House readout of Monday’s meeting did not explicitly mention the Chinese outbreak, but it did mention “the importance of manufacturers such as Sanofi and AstraZeneca working to meet demand with a sense of urgency as we head into the winter season.”
“Monday’s meeting follows numerous in-person and virtual meetings to seek ways manufacturers can make more RSV immunizations available for infants. As a result, manufacturers earlier this month committed to producing tens of thousands of additional RSV immunizations for infants and confirmed that all of the additional 77,000 doses have been released,” the White House said.
The Food and Drug Administration (FDA) in May approved the first RSV vaccine for use in the United States. The vaccine, called Arexvy, is a product of GlaxoSmithKline Biologicals (GSK), a British multinational corporation.
In November, CDC released 77,000 additional doses of Beyfortus, a monoclonal antibody to treat RSV for infants produced by Sanofi and AstraZeneca. CDC recommends immunizing infants younger than 8 months who are entering their first fall-and-winter RSV season and some older infants deemed to be at severe risk of RSV infection.
“These additional doses will be distributed immediately to physicians and hospitals through the Vaccines for Children Program and commercial channels,” CDC said, pledging to work with manufacturers to ensure there are enough doses available to meet any additional demand.
Reuters on Tuesday noted that RSV cases in the United States “began a sharp upward trend in the middle of October and were at the highest level since January last winter with 4,952 cases detected through testing in the week ended Nov. 4.”
The Hill reported that demand for the new RSV immunizations has “outpaced” what manufacturers anticipated, prompting the CDC to scale back its recommendations for administering to infants. CDC is currently advising doctors to prescribe Beyfortus only for infants with “the highest risk for severe RSV disease.”
The shortage of Bayfortus has drawn some attention from Congress. On Monday, Sen. Richard Blumenthal (D-CT) gave a speech at an East Hartford community clinic in which he said Sanofi and AstraZeneca should be held responsible for ensuring there are enough doses of the vaccine to supply both private insurance plans and the government’s Vaccines for Children program.
“Insurance should cover it. The Vaccines for Children program does cover it. But there is simply a shortage of it so many pediatricians can’t provide it for their patients,” Blumenthal said.
Blumenthal and several other Democrats sent a letter to Sanofi and AstraZeneca this month asking the companies to explain how supply problems occurred, and how it will prevent them from happening in the future.
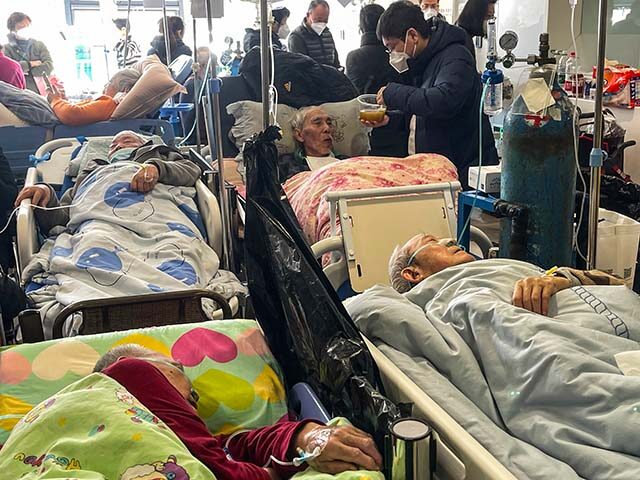
Despite China’s constant assurances that it has its own massive RSV outbreak under control, there is understandable international apprehension about another worldwide plague breaking loose from the duplicitous authoritarian regime.
The RSV season was especially severe in several countries around the world last year, including the United States, perhaps due in part to the lingering effects of the Wuhan coronavirus pandemic. Epidemiologists theorize the pandemic and its lockdowns may have disrupted the development of natural immunity, which much of the population normally acquires by contracting and defeating mild bouts of RSV during the winter cold season.
“Our immune systems completely and totally forgot about what it was,” Backus Hospital pediatric specialist Dr. William Horgan said of RSV on Monday, pithily summarizing an immunity problem that has also been cited by Chinese doctors to explain their own crisis.
RSVs are highly contagious and usually mild, although they can produce severe and even fatal illnesses in some individual, especially people with pre-existing respiratory conditions and the elderly. This year marks the first time that effective vaccines against RSV have been approved for use by the American public. With China’s outbreak and rising infections in the U.S. making news, interest in the treatments is naturally high – seemingly much higher than the drug makers anticipated.
COMMENTS
Please let us know if you're having issues with commenting.