CDC: 2.1 Million in the U.S. Have Been Vaccinated
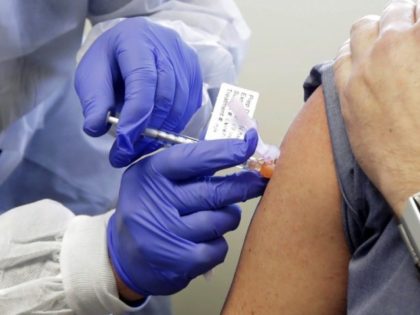
Over 2 million people in the United States have received a vaccine for the Chinese coronavirus, according to the Centers for Disease Control and Prevention (CDC).
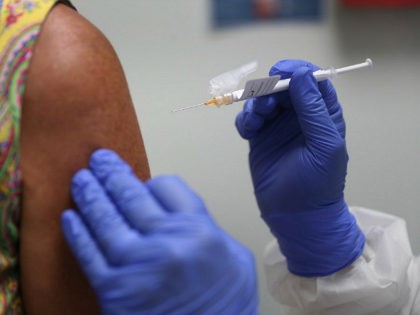
Over 2 million people in the United States have received a vaccine for the Chinese coronavirus, according to the Centers for Disease Control and Prevention (CDC).

After the injection, Harris laughed and said, “That was easy!”

President Rodrigo Duterte of the Philippines threatened to cut ties between his nation’s military and America’s – in existence since the Philippines declared independence from Spain – this weekend if Washington did not ship Manila at least 20 million doses of experimental vaccines for the Chinese coronavirus.

More than one million Americans have received a vaccine for the Chinese coronavirus, according to the Centers for Disease Control and Prevention (CDC).

China’s state-run Global Times newspaper warned Europe that purchasing American-made Chinese coronavirus vaccines was “risky” and that, without significantly transforming “the Western system and lifestyle,” policies to contain the pandemic would fail.

A CDC panel recommended persons 75 or older and frontline essential workers be the next group to get the vaccine against the coronavirus.

Several states may receive less Pfizer vaccines next week than initially promised, prompting Pfizer to blame the delay on the federal government.

A U.S. Food and Drug Administration (FDA) advisory panel on Thursday voted to recommend the emergency use approval of the Moderna vaccine.
The teams behind Britain’s Oxford/AstraZeneca vaccine and Russia’s Sputnik V vaccine will join forces to see if their coronavirus prophylactics work better in combination.

Joe Biden pledged his administration will distribute 100 million coronavirus vaccines to individuals most at risk during his first 100 days in office.

The pharmaceutical company Moderna is applying today for emergency authorization from the FDA to produce a vaccine for the coronavirus.
Monday on Fox Business Network’s “Mornings with Maria,” Moderna CEO Stephane Bancel gave an update on the coronavirus vaccine after requesting an emergency authorization of the product.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” Fauci said at a press briefing with the White House Coronavirus Task Force led by Vice President Mike Pence.

The British government has refused to rule out the notion of making Chinese coronavirus vaccines mandatory if it considers the voluntary administration of vaccines to be inadequate in slowing the spread of the virus. On Monday, Health Secretary Matt Hancock

Former Vice President Joe Biden reacted to the news of Moderna’s coronavirus vaccine, warning that an effective vaccination remains “months” away and urging Americans to continue social distancing and wearing masks.

Moderna, Inc., reported Monday that its experimental coronavirus vaccine had proven 94.5% effective in clinical trials, the latest good news in the battle against the pandemic and an affirmation of President Donald Trump’s Operation Warp Speed.

“We remain on track to deliver a vaccine before the end of the year, and maybe even before November 1,” he said. “We think we can probably have it sometime during the month of October.”

U.S. President Donald Trump’s administration entered into an agreement with American biotech company Moderna for 100 million doses of its experimental coronavirus vaccine, the White House and the drugmaker announced on Tuesday.

“I’m therefore proud to announce that this morning the Moderna has officially entered phase three already,” Trump said, noting that it was a final stage of vaccine development.

Biotech company Moderna began dosing participants Monday in phase three of the clinical trial for its coronavirus vaccine candidate.
Researchers reported Tuesday that an experimental coronavirus vaccine developed by the National Institutes of Health and Moderna Inc. is headed for its final stage of testing on July 27.
