A federal appeals court issued an order on Wednesday halting two U.S. Food and Drug Administration (FDA) actions which loosened restrictions around mifepristone, the first pill used in a two-drug chemical abortion regimen.
A three-judge panel of the conservative U.S. Court of Appeals for the Fifth Circuit ruled that the FDA’s 2016 decision to allow the abortion pill to be taken later in pregnancy is unlawful. The court said the same of the FDA’s 2021 rule change, which allowed the abortion pill to be mailed directly to patients and allowed medical professionals other than doctors to prescribe mifepristone.
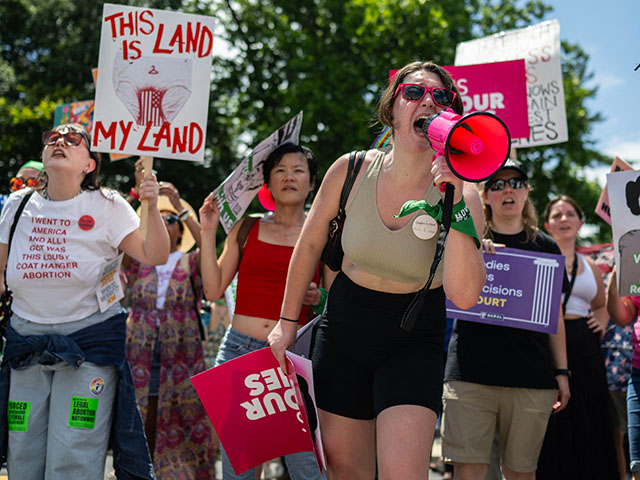
Abortion rights demonstrators rally to mark the first anniversary of the U.S. Supreme Court ruling in the Dobbs v Women’s Health Organization case in Washington, DC on June 24, 2023. (ANDREW CABALLERO-REYNOLDS/AFP via Getty Images)
“In loosening mifepristone’s safety restrictions, FDA failed to address several important concerns about whether the drug would be safe for the women who use it,” reads the panel opinion, penned by Judge Jennifer Walker Elrod.
“It failed to consider the cumulative effect of removing several important safeguards at the same time. It failed to consider whether those ‘major’ and ‘interrelated’ changes might alter the risk profile, such that the agency should continue to mandate reporting of non-fatal adverse events,” the opinion continued. “And it failed to gather evidence that affirmatively showed that mifepristone could be used safely without being prescribed and dispensed in person.”
Elrod wrote that at the preliminary stage, plaintiffs “have made a substantial showing” that the 2016 and 2021 rule changes violate the Administrative Procedure Act (APA).
Despite the court’s ruling against the government and the drug manufacturer, mifepristone will remain available for now under existing regulations while the litigation continues. The Supreme Court preemptively paused any ruling from an appeals court this spring, pending a petition for the Supreme Court to take the case. If the Supreme Court does not take the case, the Fifth Circuit’s order will go into effect.
WATCH — Pro-Life Activists Harassed by Woman Outside Family Planning Associates Building in Los Angeles:
survivors.la“Accordingly, those actions will be stayed pending final judgment. But to repeat, all of this relief is subject to the Supreme Court’s prior order, which stays the district court’s order until the disposition of any petition for certiorari,” Elrod wrote.
Judge James Ho wrote his own opinion, concurring in part and dissenting in part. Ho argued that in addition to subsequent agency action being unlawful, the FDA’s initial approval in 2000 “also violates the agency’s own rules and thus must be set aside under the APA as well.”
“The FDA approved mifepristone under its Subpart H regulations. But Subpart H only authorizes the FDA to approve drugs that ‘treat serious or life-threatening illnesses.’ And pregnancy is plainly not an illness,” Ho wrote. “So it was unlawful for the FDA to approve mifepristone under Subpart H.”
“Perhaps the FDA could have approved mifepristone through some other regulatory process,” he added. “But established precedent requires us to review the FDA’s action based on the path it took — not the path it might have taken.”
The Alliance Defending Freedom (ADF) filed the lawsuit in November of 2022 against the FDA on behalf of four national medical associations and several doctors, alleging the agency “chose politics over science and approved chemical abortion drugs for use in the United States.”
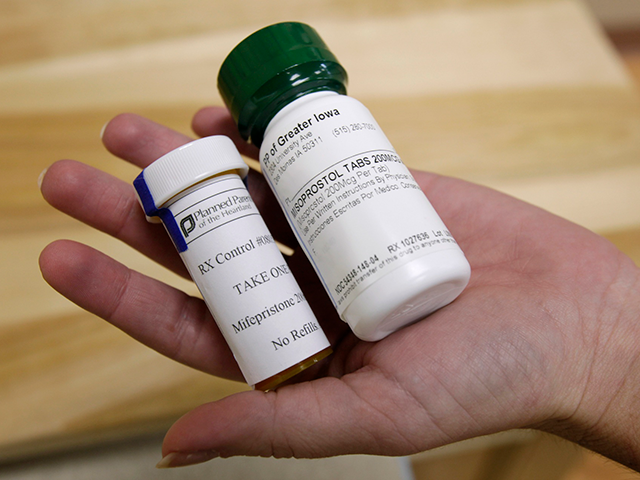
Bottles of abortion pills mifepristone, left, and misoprostol, right, at a clinic in Des Moines, Iowa. (AP Photo/Charlie Neibergall, File)
The lawsuit points to six discrete agency actions since the legalization of mifepristone and misoprostol in 2000. The ADF first filed the lawsuit in the U.S. District Court for the Northern District of Texas, and called the lawsuit the first of its kind.
The ADF alleged that the FDA never studied the safety of mifepristone under the labeled conditions of use, ignored the potential impacts of the hormone-blocking regimen on the developing bodies of adolescent girls, disregarded evidence that chemical abortion drugs cause more complications than surgical abortion, and eliminated necessary safeguards for pregnant girls and women who take the regimen.
The lawsuit also details how, in 2016, the FDA extended the permissible gestational age of the baby for which a girl or woman may take the abortion drugs — from seven weeks gestation to ten weeks gestation. Then, in 2021, the FDA allowed abortionists to send mifepristone through the mail, which the ADF says was “in direct violation of federal law.” The FDA recently made permanent its rule to allow women and girls to receive a prescription for mifepristone via telemedicine.
The complaint alleges:
All of the FDA’s actions on chemical abortion drugs — the 2000 approval, the 2016 major changes, the 2019 generic drug approval, and the two 2021 actions to eliminate the in-person dispensing requirement — failed to acknowledge and address the federal laws that prohibit the distribution of chemical abortion drugs by postal mail, express company, or common carrier. Instead, the FDA’s actions permitted and sometimes even encouraged these illegal activities.
CEO and President of ADF Kristen Waggoner celebrated the order in a statement posted to X, formerly Twitter.
“The FDA put politics over science when it stripped away meaningful safeguards on abortion drugs. Today, the Fifth Circuit agreed with ADF attorneys that those actions were lawless,” Waggoner said. “When this ruling takes effect, the FDA must restore those critical safeguards and end illegal mail-order abortions. This is a major win for women and girls and the medical professionals who serve them.”
According to former abortionist Dr. Anthony Levatino, mifepristone blocks the action of progesterone, which the mother’s body produces to nourish the pregnancy. When progesterone is blocked, the lining of the mother’s uterus deteriorates, and blood and nourishment are cut off to the developing baby, who then dies inside the mother’s womb. The drug misoprostol (also called Cytotec) then causes contractions and bleeding to expel the baby from the mother’s uterus.
The pro-abortion Guttmacher Institute found that mifepristone is used for more than half of all abortions in the United States. In 2020, the drug accounted for 53 percent of all abortions, up from 39 percent in 2017.
The case is Alliance for Hippocratic Medicine v. FDA and Danco Laboratories, No. 23-10362 in the U.S. Court of Appeals for the Fifth Circuit.
Katherine Hamilton is a political reporter for Breitbart News. You can follow her on Twitter @thekat_hamilton.
COMMENTS
Please let us know if you're having issues with commenting.