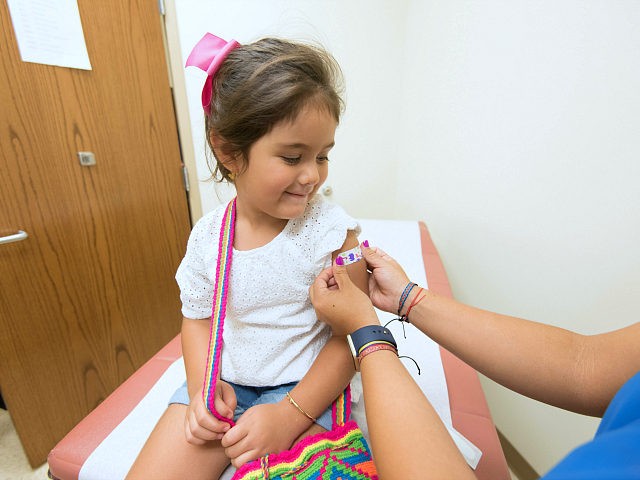
The Chinese coronavirus pandemic and the associated lockdowns disrupted pediatric outpatient visits and routine childhood vaccinations in the United States, leaving children and their communities vulnerable to potential outbreaks of preventable diseases such as measles, top health officials testified on Thursday.
In May, U.S. health officials anticipated that the spread of coronavirus and the shutdowns that restricted movement outside the home to essential activities would potentially disrupt the American health care system’s ability to continue providing routine preventive and other non-emergency care, such as vaccinations.
On Thursday, Drs. Francis Collins, the director of the National Institutes of Health (NIH), Robert Redfield, the U.S. Centers for Disease Control and Prevention (CDC) chief, and Gary Disbrow, the acting director of the Biomedical Advanced Research and Development Authority (BARDA), confirmed children and their communities are now at risk for outbreaks of vaccine-preventable diseases.
Their comments came via a joint written testimony prepared for a hearing by the Senate Appropriations Subcommittee on Health and Human Services focused on the Trump administration’s COVID-19 vaccine-acceleration program, Operation Warp Speed.
The top health officials testified:
We know from our surveys and data systems that COVID-19 interrupted access to routine medical services. CDC observed notable declines in pediatric outpatient visits and routine childhood vaccination since March, leaving some children and communities at risk for preventable disease and outbreaks. Corresponding declines were also observed in the number of measles-containing vaccine doses administered in eight U.S. health care organizations serving publicly and privately insured patients. On a positive note, however, we have started to see recovery in vaccine ordering data.
In March, California became the first U.S. state to impose a stay-at-home order to stem the spread of COVID-19. The vast majority of states followed suit.
“In order to ensure adequate hospital and medical care capacity, we must work aggressively to increase influenza and other routine childhood immunizations,” the officials testified. “Further, as we continue to fight the pandemic, it is important that Americans have confidence in all vaccines.”
In May, the CDC warned that the COVID-19 pandemic outbreak and the associated lockdowns are driving down the administration of non-influenza childhood vaccines to combat measles and other diseases.
Global public health experts, including from the World Health Organization (WHO), published data in May warning that over 80 million children in dozens of countries are potentially at risk for diseases such as measles and polio as the coronavirus pandemic ravaged routine vaccination schedules.
“CDC is working with partners to catch up and restore the high levels of immunization,” the U.S. health officials added in their joint testimony on Thursday. “Fortunately, these efforts will provide opportunities to develop innovative systems and partnerships that will pave the way for COVID-19 vaccine distribution.”
The top public health experts said they are optimistic that the Trump administration’s Operation Warp Speed would generate a safe and effective vaccine to combat COVID-19 by the end of the year or early 2022.
There are no vaccines for use against previous forms of coronavirus. Nevertheless, the officials wrote, “There is growing optimism that one or more of these vaccine candidates may prove safe and effective by late 2020 or early 2021.”
Dr. Collins, the NIH director, verbally indicated that the Trump administration would meet its target of distributing 300 million doses by early 2021.
“That’s really a stretch goal, but it’s the right goal for the American people,” he said.
The U.S. government, particularly the NIH, is also working with private companies and other countries to evaluate the safety and efficacy of COVID-19 therapeutics essential to treating patients who have been infected by the virus.
NIH components “are working concurrently with partners in academia and industry to pursue the development and testing” of several treatments.”
The potential therapeutics include monoclonal antibodies (mAbs), antivirals like remdesivir, the anti-inflammatory drug colchicine commonly used to treat gout, and anti-thrombotic drugs that reduce the formation of life-threatening blood clots caused by the coronavirus.
Moreover, the U.S. government is studying “whether convalescent plasma or blood plasma from individuals who have recovered from COVID-19, can help reduce the progression of COVID-19 in patients with mild symptoms,” the officials wrote.
NIH, CDC, and BARDA are all components of the U.S. Department on Health and Human Services (HHS).
COMMENTS
Please let us know if you're having issues with commenting.