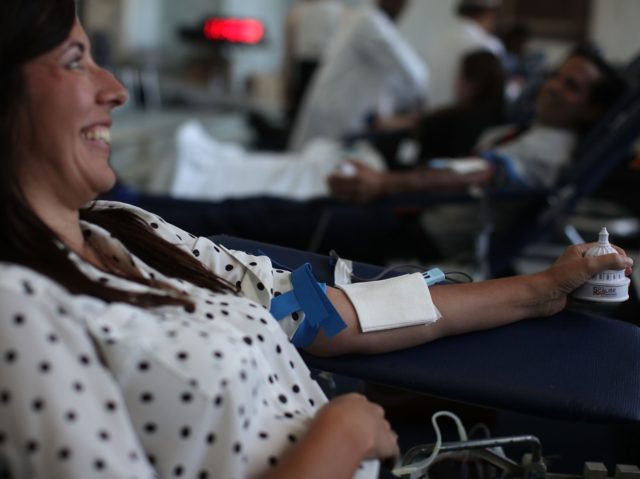
The federal Food and Drug Administration (FDA) is calling on the public to donate blood to ensure the nation has a stable blood supply as the battle against the coronavirus continues.
“We need people to start turning out in force to give blood,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in the agency’s announcement of the campaign.
The FDA launched the campaign to counter what it says is an unrealistic fear that donating blood could put one at risk for getting the virus.
“The coronavirus does not pose any known risk to blood donors during the donation process or from attending blood drives,” the announcement said.
The agency reported that over the past week blood centers across the country have experienced a significant drop in donations, which is limiting the ability to make sure the nation’s blood supply is replenished and stable.
“We need people to prevent the blood supply from getting depleted,” Marks said. “We need it not to get to the point that surgeries are having to get canceled.”
“That’s something we absolutely do not want to have happen,” Marks said. “To ensure an adequate blood supply we need people to come out and donate blood.”
Dr. Admiral Brett Giroir, assistant secretary of health at the Department of Health and Human Services, reiterated the safety of donating blood.
“It is safe to donate blood,” Girior said. “Healthy individuals should schedule an appointment to donate today to ensure that blood is available for those patients who need it.”
“Blood donors are needed now more than ever,” Katy Fry, chief executive of America’s Blood Centers, said in the FDA announcement.
The Centers represent about 50 blood centers in the U.S. and Canada, which collect almost 60 percent of the nation’s blood supply.
“We cannot wait for the situation to intensify further before taking action,” Fry said. “The blood supply cannot be taken for granted and the coronavirus only heightens the need for a ready blood supply.”
The FDA campaign reflects the nationwide trend of shutting down events, including blood drives.
“Blood drives across the country are being canceled,” Chris Hroudam president of Biomedical Services for the American Red Cross, said. “This is going to end up in an unprecedented situation if we’re not careful.”
“We are doing everything in our power to ensure that we don’t get to a critical level of the blood supply,” Hroudam said. “If we continue to see blood drives cancel, we are going to reach a level of inventory of which we haven’t seen in the past.”
Debra BenAvram, CEO of AABB, the association that accredits the majority of blood drives in the U.S., shared the need for a stable blood supply,
“Blood is an essential part of health care and the need for blood is constant,” BenAvram said. “In the United States, a patient is treated with a blood transfusion every two seconds.”
“The Armed Services Blood Program (ASBP), the official blood collector of the U.S. Military, is also echoing the call of its civilian counterparts,” the FDA announcement said.
“As the U.S. Military’s official blood program, we always have a mission to stand ready,” Col. Audra L. Taylor, ASBP Division Chief, Combat Support, Defense Health Agency, said.
“We are asking if you are able and eligible, consider donating,” Taylor said. “Take the time to help us stand mission-ready.”
The FDA has reiterated that there are no reported or suspected cases of transfusion-transmitted coronavirus and the virus poses no known risk to patients getting blood transfusions.
Blood centers in the U.S. are regulated by the FDA and must follow specific guidelines to ensure safe blood is available at all times.
Follow Penny Starr on Twitter
COMMENTS
Please let us know if you're having issues with commenting.