
Earlier this year, the FBI raided the medical offices of Dr. Salomon Melgen, seizing documents to investigate suspicions that he was overbilling Medicare. The Miami Herald reported, earlier this month, that one of the red flags that launched the Department of Health and Human Services Center for Medicare and Medicaid Services (“CMS”) investigation was Melgen’s alleged practice of dividing a single vial of the expensive liquid drug Lucentis into four doses and billing Medicare for each dose. A vial is intended by the manufacturer to generate a single dose in the treatment of macular degeneration. A medical expert told Breitbart News that Melgen’s alleged practice is highly unusual.
Dr. Michael Repka, Medical Director of Governmental Affairs of the American Academy of Opthalmology, told Breitbart News in an exclusive interview “it doesn’t seem possible you could get four doses [of Lucentis] out of a single vial. I would think that is hard to understand.” Repka noted that he was not commenting on the Melgen investigation, but was providing general information about standard practices among opthalmologists for the use of Lucentis in the treatment of macular degeneration.
Melgen’s friendship with and substantial political donations to Senator Robert Menendez (D-NJ) are at the center of an ever expanding political scandal that has prompted the New York Times to call for Menendez’s removal from the Chairmanship of the Senate Foreign Relations Committee.
Earlier this month, Menendez admitted that he spoke directly to CMS in 2009 and 2012 about Melgen’s case. “The bottom line is, we raised concerns with CMS over policy and over ambiguities that are difficult for medical providers to understand and to seek a clarification of that and to make sure, in doing so, providers would understand how to attain themselves,” Menendez told the Associated Press. But an official familiar with the CMS investigation said that on both occasions Senator Menendez urged CMS officials “to change what he called an unfair payment policy that had cost his friend Melgen $8.9 million.”
Menendez’s statement is very similar to the position taken by Melgen’s attorneys, who deny their client has done anything wrong. According to the Herald, they claim Melgen simply “differed with the program over its reimbursement policy for Lucentis.”
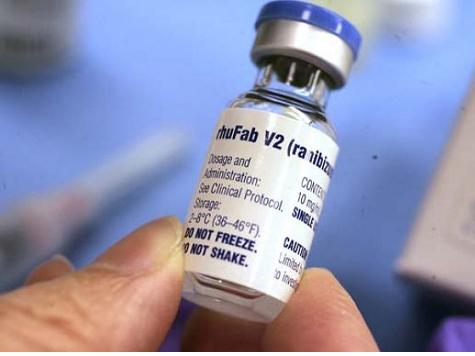
But the CMS reimbursement policy for the use of Lucentis is straightforward and not especially prone to misunderstanding on the part of opthalmologists, who use two drugs for the treatment of macular degeneration. Lucentis, which costs doctors $2,000 per vial, is FDA approved for use in treatment of macular degeneration. Avastin, which costs doctors a mere $50 per vial, is FDA approved, but not yet for the treatment of macular degeneration. Both drugs are manufactured by biotech giant Genentech.
CMS has established the billing protocol for opthalmologists who purchase either Avastin or Lucentis for use in the treatment of patients with macular degeneration. Doctors are allowed to bill Medicare the “average selling price” of the medicine plus 6 per cent. In the case of a patient treated with a single vial of Lucentis, the opthalmologist would bill Medicare $2,000 plus 6%, or $2,120 for the medicine. When the doctor uses Avastin instead of Lucentis, the bill submitted to Medicare would be $50 plus 6%, or $53.
The Herald reported that Dr. Melgen uses Lucentis to treat macular degeneration patients “more than any other ophthalmologist in Florida and possibly the country. His high patient volume also raised red flags for investigators. ” That high usage prompted the formal investigation focused on potential Medicare overbilling. Specifically, the Herald reported that “Melgen is suspected of using one vial for as many as four patients, while submitting claims of up to $8,000, as if he had used separate vials for each, according to sources familiar with the billing dispute.”
Instead of billing Medicare at a rate equal to the “average sale price” plus 6% for the approved use of a single vial (which would have generated a reimbursement of $2,120), Melgen is suspected of squeezing four doses out of a single vial, and billing Medicare $8,000 plus 6%, or $8,480. Doctors who play by the straightforward CMS billing policy make a $120 profit on each vial of Lucentis they purchase. A doctor who violates the CMS billing policy and charges $8,480 would make a $6,480 profit on each vial of Lucentis they purchase.
Many opthalmologists choose to use Avastin rather than Lucentis for the treatment of their patients with macular degeneration, and are upset that Genentech has failed to apply for FDA approval for Avastin as a treatment for macular degeneration. They argue that the drug is as effective as Lucentis, and at 2.5% of the cost provides patients an affordable treatment.
Medical research is indeterminate as to the merits of Avastin versus Lucentis in the treatment of macular degeneration. In practice, most opthalmologists use both, since individual patients have different reactions to each drug. For some, Avastin is more effective. For others, Lucentis is a superior solution.
“These drugs–Lucentis and Avastin– have been vision saving for the public,” Dr. Repka noted. “We are several years into Lucentis. The FDA initially approved it for use in the treatment of macular degeneration on June 30 2006. Avastin is not FDA approved for this indication.”
Manufacturers package the liquid medicine in small vials. Their intention is to include the amount of a single dose, plus a small percentage of “overfill,” designed to make sure that a full single dose can be obtained in the case of spillage. Each vial of Lucentis contains a tiny amount–.05 milliliters– of medicine in liquid form. As the manufacturer’s literature states, it “is a sterile, colorless to pale yellow solution in a single-use glass vial. [It] is supplied as a preservative-free, sterile solution in a single-use glass vial.”
The treatment consists of drawing the liquid out of the vial into a syringe, inserting the syringe into the eyeball of the patient, then emptying the liquid contents of the syringe into the inside of the patient’s eye.
In a case of one arm of government acting in opposition to another arm of government, the inspector general of the Department of Health and Human Services issued a report in 2012 that recommended Medicare officials should stop reimbursing for Lucentis “because there was a cheaper and equally effective alternative.”
In any event, it seems implausible that Melgen’s alleged overbilling of Medicare stems from confusing or conflicting rules, as Menendez’s office claims. The guidelines for reimbursing doctors for the use of drugs to treat macular degeneration are straightfoward. Melgen’s alleged practices, however, would be very much less than that.
COMMENTS
Please let us know if you're having issues with commenting.