Orthofix Announces FDA Clearance and First Patient Implants of FIREBIRD SI Fusion System for Low Back Pain
Orthofix Announces FDA Clearance and First Patient Implants of FIREBIRD SI Fusion System for Low Back PainThe Associated PressLEWISVILLE, Texas
LEWISVILLE, Texas–(BUSINESS WIRE)–Jun 29, 2020–
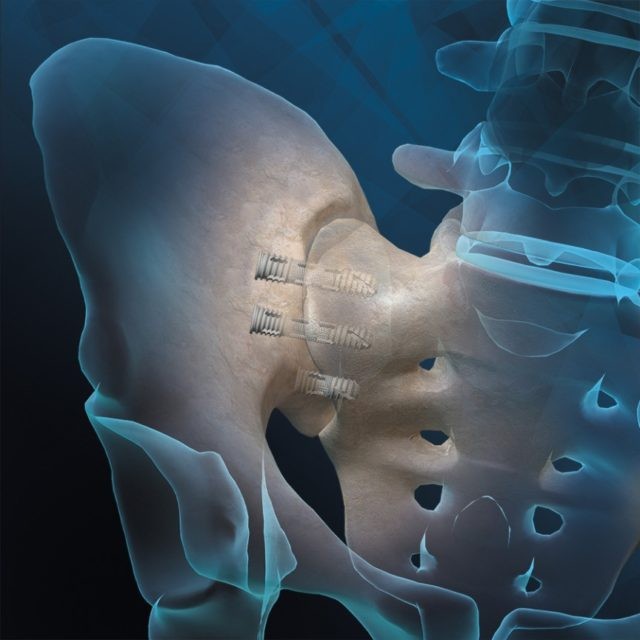
Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company focused on musculoskeletal healing products, announced the U.S. Food and Drug Administration (FDA) 510(k) clearance and the first patient implants of the FIREBIRD ™ SI Fusion System. Designed to compress and stabilize the sacroiliac joint (also called the SI joint) during fusion, the FIREBIRD SI Fusion System is the first 3D printed titanium bone screw to launch in the U.S. for treating SI joint dysfunction.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200629005121/en/
Illustration of the Orthofix FIREBIRD SI Fusion System for Low Back Pain (Photo: Business Wire)
“Pain in the lower back and buttocks may often be caused by degeneration of the SI joint as a result of stress to the joint during pregnancy or childbirth, everyday normal wear and tear, or an injury such as a fall,” said Dr. Justin Hall, an orthopedic surgeon who implanted the first patients at Baptist Memorial Hospital-Golden Triangle in Columbus, MS. “Sacroiliac joint fusion is an important treatment option to reduce pain through a minimally invasive procedure that can enable patients to return to better function and quality of life.”
The FIREBIRD SI Fusion System is designed with a porous mid-shaft region that allows bone to grow into its surface – creating a bond between the implant and the patient’s bone at the SI joint. Featuring a cannulated screw design, the system enables the surgeon to pack the device with autograft and/or allografts like the Trinity ELITE ™ Allograft with viable cells to help ensure bone fusion. The FIREBIRD SI screws are available in an assortment of lengths and diameters to address a variety of patient anatomies. This novel system is being launched in the U.S through a limited market release.
“Orthofix Spine prides itself in creating differentiated technology for the advancement of patient care,” said Kevin Kenny, Global President of Orthofix Spine. “We are excited to bring the FIREBIRD SI Fusion System to market as an extension of our flagship FIREBIRD line of devices. This new system is another example of our commitment to providing surgeons and their patients with innovative options that interface with products and solutions within our portfolio.”
The FIREBIRD SI Fusion System is implanted through a minimally invasive surgery that typically can be performed in about an hour and may lead to less time at the hospital and a faster recovery than a traditional sacroiliac joint fusion surgery. The procedure with the FIREBIRD SI Fusion System involves inserting two to four bone screws across the SI Joint to stabilize during the fusion process.
About SI Joint Pain
The sacroiliac joint connects the hip bones to the sacrum. Covered with cartilage, the SI joints sit on each side of your spine and allows for some movement. They act as a shock absorber between the upper body and the pelvis and legs. Dysfunction of the SI joint may cause low back and/or leg pain that is typically worse when standing and walking. Published clinical literature indicates that sacroiliac joint pain is estimated to affect between 15 and 30 percent of individuals with chronic low back pain.
Common causes that may lead to SI joint dysfunction and pain include trauma, lifting or twisting, pregnancy, natural childbirth, degeneration from previous lumbar spine surgery, stresses to the joint due to leg length differences, joint replacement or scoliosis among others.
About Orthofix
Orthofix Medical Inc. is a global medical device company focused on musculoskeletal products and therapies. The Company’s mission is to improve patients’ lives by providing superior reconstruction and regenerative musculoskeletal solutions to physicians worldwide. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedic extremities products are distributed in more than 70 countries via the Company’s sales representatives and distributors. For more information, please visit http://www.orthofix.com.
Forward Looking Statements
This communication contains certain forward-looking statements under the Private Securities Litigation Reform Act of 1995. These forward-looking statements, which may include, but are not limited to, statements concerning the estimates, projections, financial condition, results of operations and businesses of Orthofix and its subsidiaries, are based on Orthofix management’s current expectations and estimates and involve risks and uncertainties that could cause actual results or outcomes to differ materially from those contemplated by the forward-looking statements.
The forward-looking statements in this release do not constitute guarantees or promises of future performance. Factors that could cause or contribute to such differences may include, but are not limited to, risks relating to: practices of health insurance companies and other third-party payors with respect to reimbursement for our devices and other risks described in the “Risk Factors” section of our 2019 Annual Report on Form 10-K, as well as in other reports that we file in the future. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The Company undertakes no obligation to update or revise the information contained in this press release.
View source version on businesswire.com:https://www.businesswire.com/news/home/20200629005121/en/
CONTACT: Mark Quick
Investor Relations
Tel 214 937 2924
markquick@orthofix.comDenise Landry
Media Relations
Tel 214 937 2529
KEYWORD: UNITED STATES NORTH AMERICA TEXAS
INDUSTRY KEYWORD: MEDICAL DEVICES FDA HEALTH SURGERY OTHER HEALTH BIOTECHNOLOGY
SOURCE: Orthofix Medical Inc.
Copyright Business Wire 2020.
PUB: 06/29/2020 07:00 AM/DISC: 06/29/2020 07:01 AM
COMMENTS
Please let us know if you're having issues with commenting.