A first delivery of mpox vaccines is expected to arrive in the Democratic Republic of Congo in the coming days, the World Health Organization said Friday.
The WHO declared an international emergency over mpox on August 14, concerned by the surge in cases of the new Clade 1b strain in the DR Congo that spread to nearby countries.
After returning from the DRC on Friday, WHO chief Tedros Adhanom Ghebreyesus told a press conference: “We hope to have the first delivery in the next few days, and then it will build up.”
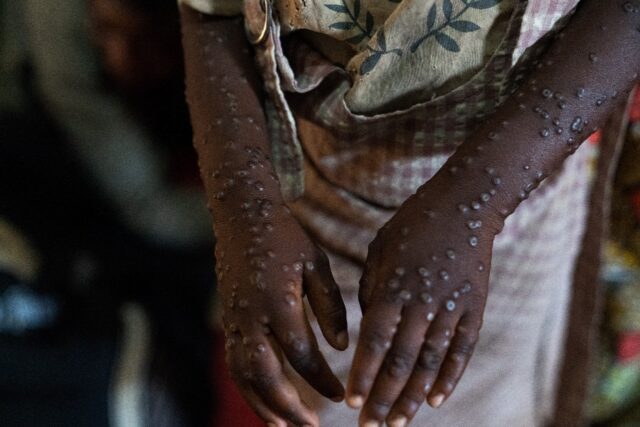
More than 18,000 suspected cases of mpox have been reported in the DRC so far this year, with 629 deaths, said Tedros.
The figure included more than 5,000 cases and 31 deaths in the eastern provinces of North and South Kivu, where Clade 1b has been spreading.
“The number of reported cases of Clade 1b has been rising rapidly for several weeks. Fortunately, relatively few deaths have been reported in recent weeks,” said Tedros.
In addition, 258 cases of Clade 1b have been confirmed in neighbouring Burundi; four in Rwanda, four in Uganda, two in Kenya and one each in Sweden and Thailand, he added.
In the DRC, Tedros met with President Felix Tshisekedi to discuss the outbreaks, the importance of clear communication on the virus and strong engagement with local communities.
“We believe we can stop these outbreaks in the next six months,” the UN health agency’s director-general said.
Procuring vaccines for DRC
WHO Emergency Use Listing (EUL) is designed to expedite the availability of unlicensed medical products, such as vaccines, for use in a public health emergency.
The two main procurers of vaccines for low-income countries — the Gavi vaccine alliance and the UN children’s agency UNICEF — require EUL status to buy vaccines for use in countries that have not issued their own national regulatory approval.
Two mpox vaccines — MVA-BN, produced by Danish drugmaker Bavarian Nordic, and Japan’s LC16 — have been put forward for EUL status.
Tedros said the information the WHO had hitherto on the two vaccines was partial, and the UN health agency received the additional information it needed on August 23.
The process for granting EUL could take another two weeks, but in the meantime, Tedros has given Gavi and UNICEF the authority to begin procuring vaccines pending approval.
“We don’t need to wait until two weeks before we proceed with procurements,” he explained.
However, “the safety and efficacy of vaccines are our highest priority. We will not take shortcuts,” he added.
Donations and pledges
Tim Nguyen from the WHO health emergency programme said the European Union had procured 175,000 doses of MVA-BN, while Bavarian Nordic was donating 40,000 doses.
All in all, there are “about 230,000 doses that we understand are imminently available to be dispatched to affected regions”, he said.
There were further pledges of donations, but Nguyen said they needed to materialise into donations.
Maria Van Kerkhove, the WHO’s epidemic and pandemic preparedness and prevention director, said vaccines were only one of several available tools to crack down on the virus.
“Right now, even when vaccines aren’t available — and it’s going to take some time for the vaccines to reach those at risk — the right messages can be reaching the right at-risk populations,” she said.
COMMENTS
Please let us know if you're having issues with commenting.