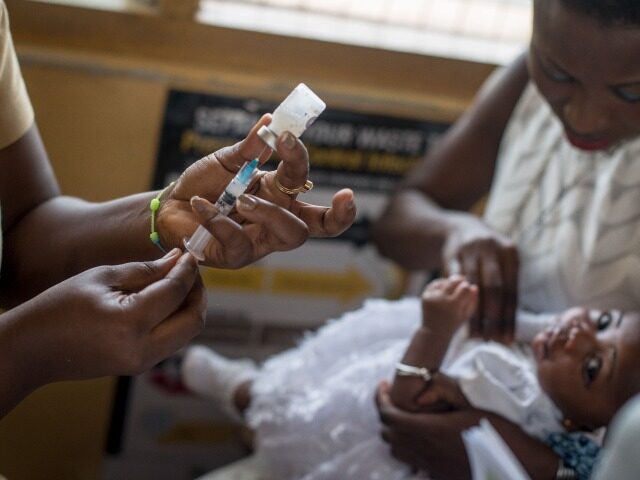
Ghana on Wednesday became the first country to approve the use of the R21/Matrix-M malaria vaccine developed by Oxford University.
The vaccine, which studies indicate is far more effective than any previously approved for human use, has been described as a “world-changer” by the scientists who created it.
R21 is actually the second malaria vaccine to be approved by the World Health Organization (W.H.O.). The first was a product called Mosquirix, developed by British pharmaceutical titan GSK. It has already been administered to over a million children in Africa, but deployment was stalled by a lack of funding and the relatively high cost of each complete inoculation which requires four doses of the vaccine per patient.
GSK plans to produce 15 million doses per year of each vaccine, but W.H.O. plans to inoculate at least 25 million African children, a goal that would require 100 million doses of Mosquirix at four doses per child.
W.H.O. issued a report last month that credited Mosquirix with reducing child mortality from all causes by about ten percent in areas where it has been deployed.
Oxford’s initial trials showed R21 is more effective than Mosquirix – up to 80 percent, versus 60 percent for the GSK product – and the cost per patient is lower, with only a single booster shot required. Oxford has a deal with the Serum Institute of India (SII) to manufacture at least 100 million doses a year, and SII said it could double that production if needed.
“The vaccine has been approved for use in children aged 5-36 months, the age group at highest risk of death from malaria. It is hoped that this first crucial step will enable the vaccine to help Ghanaian and African children to effectively combat malaria,” Oxford University said when announcing Ghana’s approval.
Professor Adrian Hill, director of Oxford’s Jenner Institute, said R21 was the “culmination of 30 years of malaria vaccine research at Oxford with the design and provision of a high efficacy vaccine that can be supplied at adequate scale to the countries who need it most.”
Hill told Reuters on Friday that R21 was the first major vaccine to be approved by an African country ahead of the richer nations in the West and Asia.
“Particularly since [the Wuhan coronavirus pandemic], African regulators have been taking a much more proactive stance, they’ve been saying … we don’t want to be last in the queue,” Hill said.
The scientific community worldwide has labored for a century to create an effective vaccine against malaria, which kills an estimated 600,000 victims per year, mostly children in sub-Saharan Africa. According to W.H.O., Ghana suffered about 5.3 million malaria infections and 12,500 deaths in 2021.
W.H.O. set the goal of creating a vaccine that is at least 75 percent effective in children, a threshold R21 clears according to trials conducted in Burkina Faso last year. A large Phase III trial is currently underway in Burkina Faso, Kenya, Mali, and Tanzania.
Delese Darko, CEO of the Ghanaian Food and Drugs Authority (FDA), said the vaccine rollout would be supervised by her country’s health service, malaria program, and immunization authority, the Expanded Program on Immunization (EPI). Darko did not provide a timetable for the rollout.
Reuters noted that Ghana normally relies on international organizations for vaccine funding, and those organizations are generally reluctant to provide funding until W.H.O. approves a new product. However, Ghanaian Managing Director of Vaccine Markets Dr. Derrick Sim said his government might have enough money to finance R21 distribution on its own, especially if SII can meet its pricing objective of less than $3 per shot.
COMMENTS
Please let us know if you're having issues with commenting.